|
 |
- Search
| Gyne Robot Surg > Volume 2(1); 2021 > Article |
|
Abstract
Precise, meticulous suture with enough strength is the most important procedure in myomectomy, as it reduces bleeding and the risk of uterine rupture in subsequent pregnancy; however it has been considered to be the most difficult task in laparoscopy. Since the da Vinci® robotic platform was introduced, robotic myomectomy gradually has become popular. It provides for EndoWrist instrumentation with favorable ergonomics allowing for rotation of instruments up to 45°; additionally, it enables surgeons to effectively enucleate myomas and achieve precision multi-layer closure. Several studies have reported that the da Vinci® robotic platform is a feasible treatment option for myomectomy. In this review, we will discuss the current status of robotic myomectomy with a focus on its differences with open myomectomy and laparoscopic myomectomy.
Laparoscopic suturing is technically challenging with a steep learning curve. One of the novel technical advances in laparoscopic surgery has been the adoption of the da Vinci® robotic surgical platform (Intuitive Surgical, Inc., Sunnyvale, CA, USA). Since the da Vinci® robotic platform’s approval by the Food and Drug Administration, robotic surgery has been quickly adopted [1,2]. Robotic surgery more closely mimicking open surgery provides improved ergonomics, wristed instrumentation with increased freedom of movement, minimized instrument tremor, depth perception, and 3-dimensional visualization [2]. It is especially advantageous for difficult procedures, such as gynecologic surgery, in which extensive dissection and anatomical reestablishment is required [1]. There are also weaknesses of the robotic platform, such as decreased tactile feedback and high costs. Also, a docking procedure which is to move robot to patient and attach all arms to the patient is necessary, which makes surgery longer. In our previous study, mean docking time was 2.4±1.6 minutes [3]. Currently, the robotic platform is widely utilized in many major gynecologic surgeries including hysterectomy, oophorectomy, myomectomy, ovarian cystectomy, and sacrocolpopexy [1].
Myomectomy is suture-intensive surgery. Precise and strong suturing is essential to reduce the risk of uterine rupture in subsequent pregnancy. Suturing is simple and easy with the robotic platform. It provides for EndoWrist instrumentation with favorable ergonomics allowing for rotation of instruments; the needle driver for multi-port da Vinci surgery can twist 540°, while the movement angle of the single-site wristed needle driver is only 45°. Indeed, two studies have reported faster suturing times for the robotic platform than in the case of laparoscopy [4,5]. Additionally, the robotic platform enables surgeons to effectively enucleate myomas and achieve precision multi-layer closure [6,7]. For these reasons, robotic myomectomy (RM) has become increasingly popular as a fertility-sparing treatment option for myomectomy.
In this review, we will discuss the current status of robotic surgery in myomectomy with a focus on its differences with open myomectomy (OM) and laparoscopic myomectomy (LM).
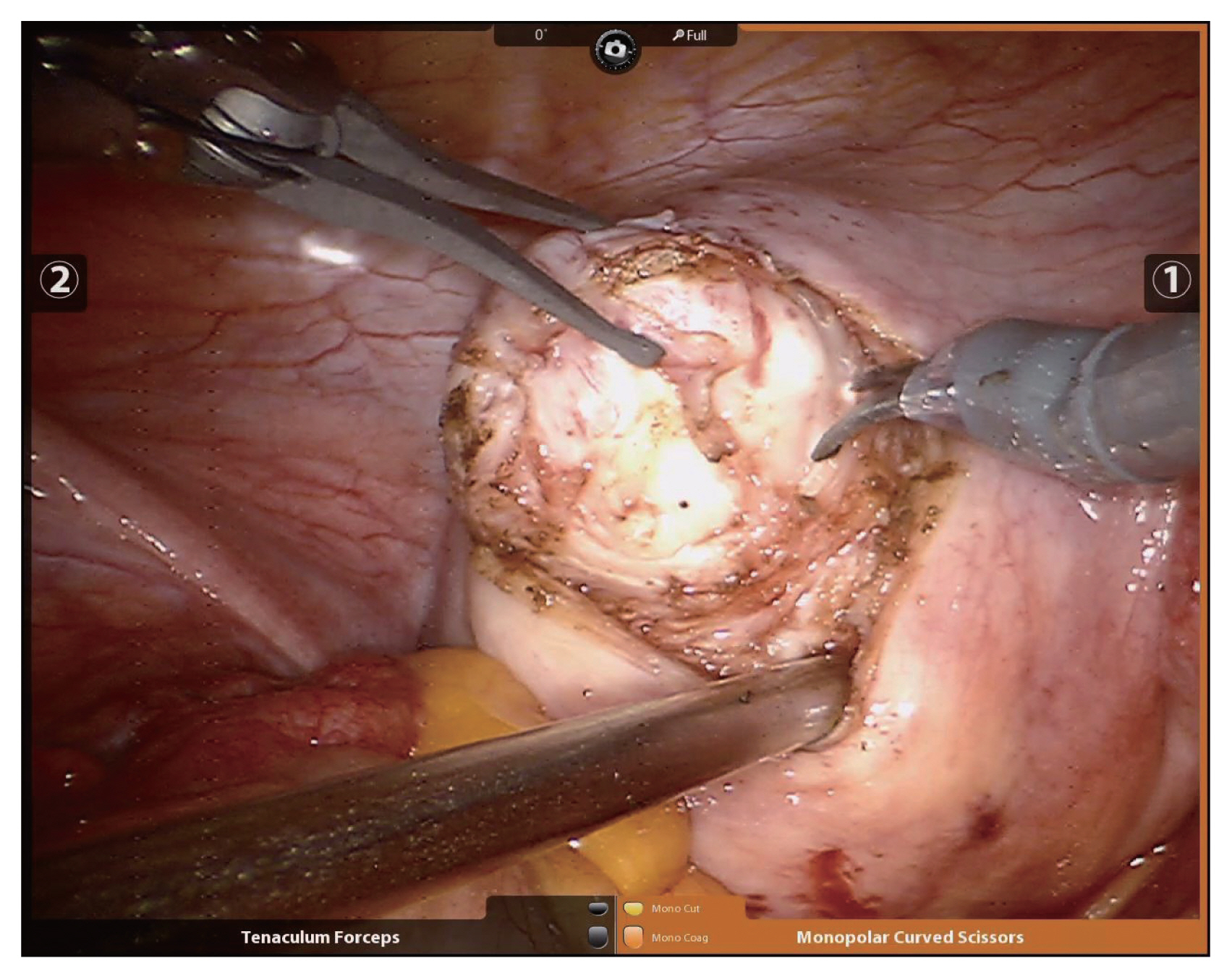
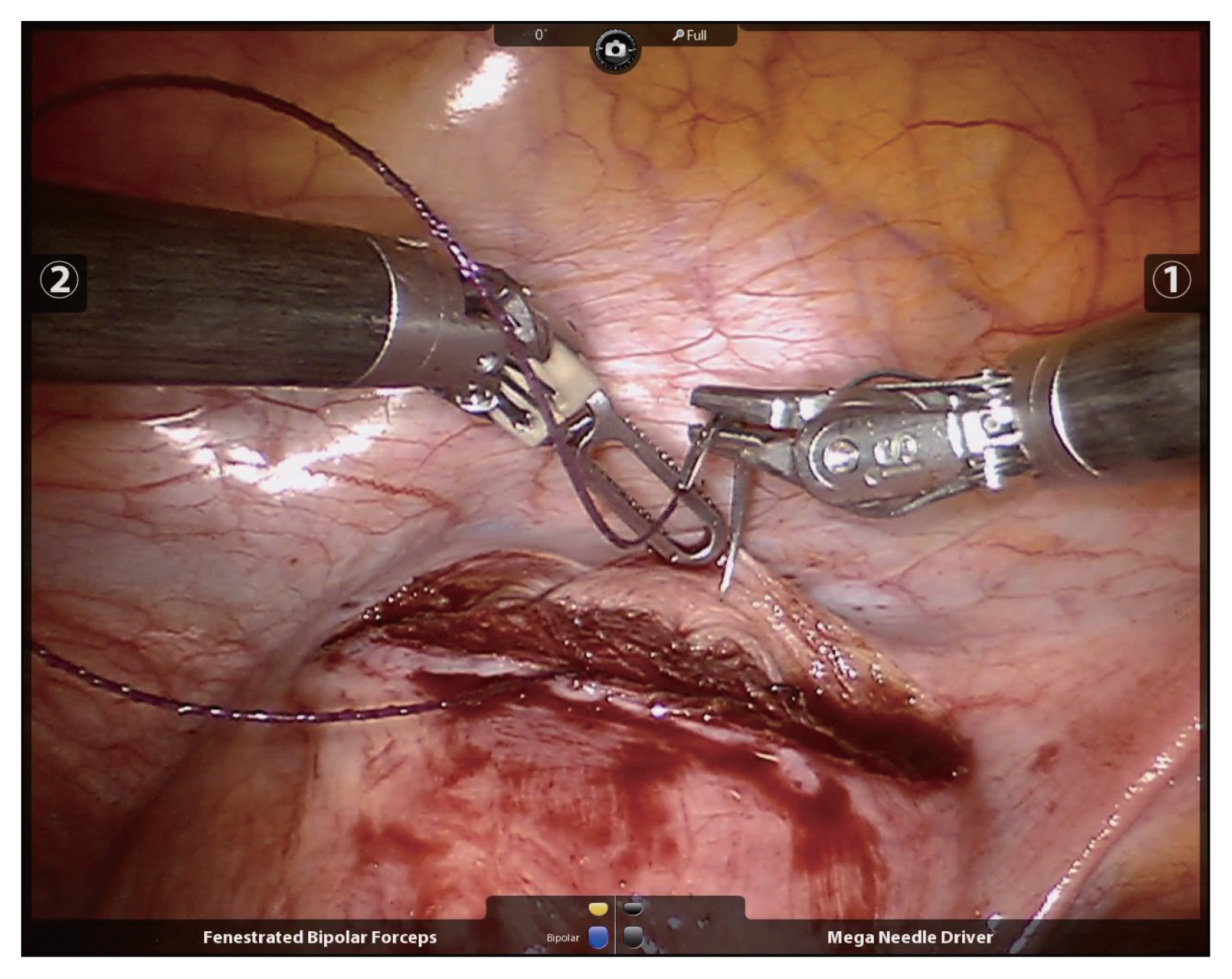
Since Advincula et al. [6] reported the first RM in 2004, several studies on RM have been reported. Basically, RM is performed using the da Vinci® Si or Xi system with multiple surgical incisions. Generally, four sites are needed, including the 12-mm camera port at the umbilicus, two 8-mm side ports, and a 12-mm or 5-mm assistant port. The assistant port is used by the assistant with laparoscopic instruments such as laparscopic irrigation suction set or a laparoscopic myoma screw or laparoscopic allis grasping forceps. Also, through the assistant port, suture material can be inserted or pulled. The uterine incision is made with a monopolar electrode in one robotic arm while counter-traction is applied using a tenaculum forceps in the other (Fig. 1). The uterine wall is sutured continuously using barbed suture material with a wristed needle holder (Fig. 2). After the suturing, enucleated myomas are retrieved by scalpel or power morcellation. This procedure has some inherent technical concerns. For example, finding small myomas not visible with the robotic system might be difficult. Indeed, whereas invisible myomas can be found by palpation during laparotomy, deep and small myomas can be missed by the robotic system, due to its lack of any functional tactility. Kim et al. [8] stated that to solve this problem, intraoperative sonographic navigation can be utilized. If such is unavailable, portable sonography can be performed after undocking the robotic system, but this is very cumbersome work. Alternatively, preoperative magnetic resonance imaging can be very helpful in RM. The robotic system can be particularly effective in dealing with deep intramural or submucosal myomas. Because of the improved 3D view and favorable ergonomics it affords, endometrium damage and incision size can be minimized during enucleation of myomas. Also, the robotic tenaculum with articulation provides for strong traction in the desired direction, which functionality facilitates removal of deep myomas. This advantage should be studied more in future.
Compared with open surgery, laparoscopic surgery affords shorter hospital stays, faster recovery as well as less adhesion formation and bleeding [9]. These advantages are also available in robotic surgery. Advincula et al. [10] reported the first comparative retrospective study between robotic and OM in 2009. They argued that although the robotic approach costs more, its decreased blood loss (195.69±228.55 vs. 364.66±473.28 mL), shorter hospital stay (1.48±0.95 vs. 3.62±1.50 days) and lower complication rates outweigh the financial burden [10]. Since then, several retrospective studies [11–15] comparing RM with OM have been published. They reported, similarly, longer operative times, shorter hospital stays, and comparable or lesser blood loss in RM than in OM. Recently, a meta-analysis found that whereas RM was inferior to OM in total operative time (84.85 minutes per operation; 95% confidence intervals [CI], 60.41–109.29), it was superior in estimated blood loss (92.78 mL per operation; 95% CI, 47.26–138.29), the need for transfusion (odds ratio [OR], 0.20; 95% CI, 0.09–0.43), total complications (OR, 0.31; 95% CI, 0.11–0.87), and length of hospital stay (1.84 days per patient; 95% CI, 1.40–2.29) [16]. There are as yet no comparative studies regarding fertility outcomes with RM and OM. To date, there are no specific criteria for selection of RM or OM.
Generally, when numerous myomas were suspected, OM was preferred over RM. Kim et al. [8] reported on 13 women who had each undergone removal of 10 or more myomas by RM, and compared the results with OM. Although RM certainly can be considered to be feasible for numerous myomas, the operative time has been reported to be twice as long as in OM (360.5 vs. 183.8 minutes; P=0.001) [8]. In summation, a minimally invasive approach including the use of both RM and LM has been shown to be associated with longer operative time, less blood loss, less need for blood transfusion and shorter hospital stay relative to OM [16].
Although there are as yet no randomized trials, several retrospective studies [13,15,17–20] comparing surgical outcomes between RM and LM have been published. Almost all of them reported comparable surgical outcomes except for operative time [13,15,17,19–21]. Nezhat et al. [17] determined that operative time was longer in RM than in LM (234 vs. 203 minutes, P=0.03), due to the needs to dock and undock the robot and to substitute instrumentation for the robotic arms. Gargiulo et al. [19] likewise reported a longer operative time in RM than in LM (195.1 vs. 118.3 minutes, P<0.001). On the other hand, other studies found that operative times were similar [3,18,22]. A meta-analysis including 15 studies concluded that there were no significant RM-versus-LM differences in surgical outcomes including operative time, estimated blood loss, transfusion rate, length of hospital stay or complication rate [16]. Note though, that these are short-term outcomes. What about long-term surgical outcomes such as recurrence and fertility rates? As for the latter, there are a few studies. Lönnerfors and Persson [23] conducted a prospective study that indicated a 68% pregnancy rate following RM. Pitter et al. [24] and Huberlant et al. [25] reported 50.8% and 52.8% pregnancy rates, respectively, following RM. Meanwhile, pregnancy rates following LM have been found to range from 50 to 60% [7]. There is still no comparative study of fertility outcomes between RM and LM.
Robotic suturing is easier and faster than laparoscopic suturing. Specifically, two studies have reported a faster suturing time for robotic suturing relative to laparoscopy [4,5]. Also, the use of the robotic tenaculum affords greater traction strength in the desired direction for deep or huge myoma compared with the laparoscopic tenaculum. However, there are as yet no studies reporting a shorter total operative time for RM versus LM. Another issue is the need to consider the benefits of robotic surgery with respect to surgery ergonomics. Lundon et al. [26] reported, based on a survey study, that robotic surgery affords notably less operator morbidity than laparoscopic surgery. Cost-effectiveness should be carefully considered prior to platform selection though, as RM is much more expensive than LM.
In summation, there seem to be no significant differences between RM and LM in short-term surgical outcomes; as for long-term outcomes, further comparative studies on pregnancy rates, complications relating to pregnancy, and recurrence rates are needed. Currently, researchers in Taiwan are conducting the first randomized control study (registered at ClinicalTrials.gov: NCT04282863) evaluating RM and LM outcomes.
The robotic single-site system refers to the da Vinci® Si or Xi system that proceeds via umbilical incision through the da Vinci® single-site® silicon port. This system allows for only a limited range of motion and limited triangulation with semi-rigid instruments [27]; indeed, there are few reports on RSSM [28,29]. Recently, Kim et al. [29] reported 101 consecutive cases for which RSSM was judged to be a feasible therapeutic option. However, due to its semi-rigid (not rigid) instrumentation to be used, some limitations were found. First, the semi-rigid instruments limited the traction on myomas [27]. In single-site robotic surgery, the counter-traction force on the myoma is applied using the semi-rigid instruments with limited angle of motion, therefore the traction force is not enough for myomectomy. Consequently, limited instrumentation with insufficient traction strength was used to provide counter-traction to the myomas. Therefore, a hybrid technique overcoming the traction-strength problem has been suggested [30]. Also, reduced-port RM schemes whereby the single-site platform is used with one conventional robotic port have been proposed [31,32]. The first one was shown to enable increased articulation and instrumental dexterity by the added conventional robotic port [31].
There have also been two comparative studies on RSSM and conventional RM [27,33]. Moawad et al. [27] reported that robotic single-site and conventional multi-port robotic myomectomies had comparable estimated blood loss (83.3 vs. 109.2 mL, P=0.34) and operative times (162.4 vs. 162.4 minutes, P=0.99), and concluded, thus, that RSSM can be considered to be of equivalent utility for selected patients. However, notwithstanding the several reports of RSSM’s feasibility, most surgeons tend to select conventional RM over RSSM due to the latter’s inherent problems and difficulties noted above.
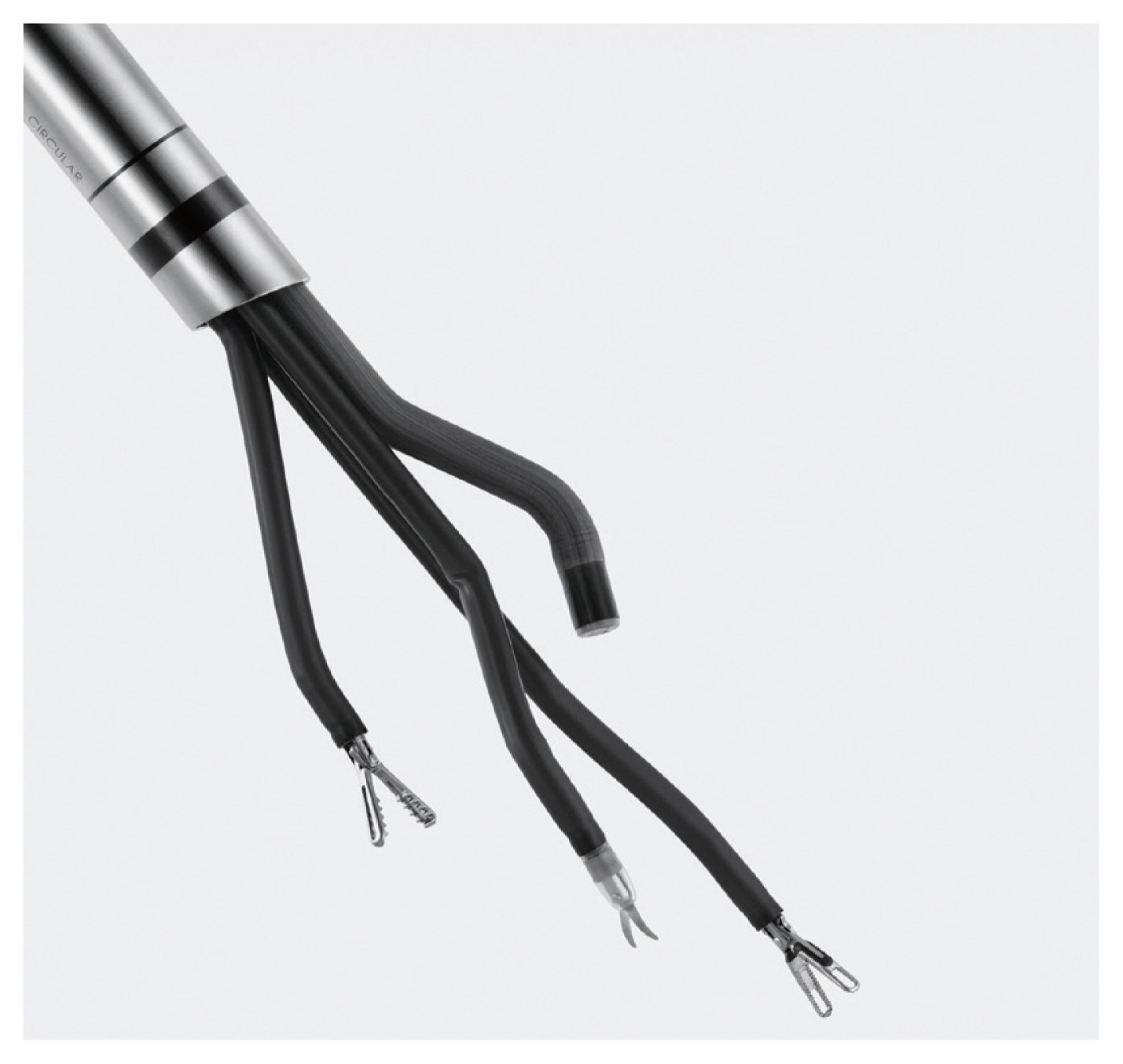
Recently, the da Vinci® SP system, designed exclusively for single-site surgery, was introduced (Fig. 3). It provides for application of three fully-wristed, elbowed instruments through the umbilical incision to overcome the drawbacks of the robotic single-site system. Also, its EndoWrist articulating camera both provides more vision and overcomes the instrument-collision problem. The initial report on myomectomy with the SP system was that of Shin et al. [34] earlier this year, which included 12 successful cases of myomectomy. They concluded that the SP system provided more power and freer access to the target area compared with the semi-rigid instrumentation of the Si or Xi single-site system [34]. However, in the SP system, instruments such as the robotic tenaculum, which provides for sufficient traction strength, are unavailable; on this basis, we supposed that the da Vinci® SP system relative to the conventional multi-port robotic Si or Xi system might not be particularly efficacious in the myomectomy setting.
The robotic platform, as compared with the other platforms, is a feasible treatment option for myomectomy. However, the available evidence does not suggest that it is superior to laparoscopy in terms of surgical outcomes, and certainly, some technical limitations remain. Long-term outcomes such as recurrence rates and fertility have not been sufficiently evaluated, either. We expect that further long-term prospective studies will clarify any possible advantages of RM.
References
1. Sinha R, Sanjay M, Rupa B, Kumari S. Robotic surgery in gynecology. J Minim Access Surg 2015;11:50–9.



3. Won S, Lee N, Kim M, Kim MK, Kim ML, Jung YW, et al. Comparison of operative time between robotic and laparoscopic myomectomy for removal of numerous myomas. Int J Med Robot 2020;16:1–5.


4. Chandra V, Nehra D, Parent R, Woo R, Reyes R, Hernandez-Boussard T, et al. A comparison of laparoscopic and robotic assisted suturing performance by experts and novices. Surgery 2010;147:830–9.


5. Narula VK, Watson WC, Davis SS, Hinshaw K, Needleman BJ, Mikami DJ, et al. A computerized analysis of robotic versus laparoscopic task performance. Surg Endosc 2007;21:2258–61.


6. Advincula AP, Song A, Burke W, Reynolds RK. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc 2004;11:511–8.


8. Kim H, Shim S, Hwang Y, Kim M, Hwang H, Chung Y, et al. Is robot-assisted laparoscopic myomectomy limited in multiple myomas?: a feasibility for ten or more myomas. Obstet Gynecol Sci 2018;61:135–41.


9. Falcone T, Bedaiwy MA. Minimally invasive management of uterine fibroids. Curr Opin Obstet Gynecol 2002;14:401–7.


10. Advincula AP, Xu X, Goudeau S 4th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol 2007;14:698–705.


11. Ascher-Walsh CJ, Capes TL. Robot-assisted laparoscopic myomectomy is an improvement over laparotomy in women with a limited number of myomas. J Minim Invasive Gynecol 2010;17:306–10.


12. Sangha R, Eisenstein DI, George A, Munkarah A, Wegienka G. Surgical outcomes for robotic-assisted laparoscopic myomectomy compared to abdominal myomectomy. J Robot Surg 2010;4:229–33.


13. Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol 2011;117(2 Pt 1):256–65.


14. Nash K, Feinglass J, Zei C, Lu G, Mengesha B, Lewicky-Gaupp C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet 2012;285:435–40.


15. Gobern JM, Rosemeyer CJ, Barter JF, Steren AJ. Comparison of robotic, laparoscopic, and abdominal myomectomy in a community hospital. JSLS 2013;17:116–20.



16. Iavazzo C, Mamais I, Gkegkes ID. Robotic assisted vs laparoscopic and/or open myomectomy: systematic review and meta-analysis of the clinical evidence. Arch Gynecol Obstet 2016;294:5–17.


17. Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy--a retrospective matched control study. Fertil Steril 2009;91:556–9.


18. Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol 2009;201:566.e1–5.


19. Gargiulo AR, Srouji SS, Missmer SA, Correia KF, Vellinga TT, Einarsson JI. Robot-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy. Obstet Gynecol 2012;120(2 Pt 1):284–91.


20. Hsiao SM, Lin HH, Peng FS, Jen PJ, Hsiao CF, Tu FC. Comparison of robot-assisted laparoscopic myomectomy and traditional laparoscopic myomectomy. J Obstet Gynaecol Res 2013;39:1024–9.


21. Lee CY, Chen IH, Torng PL. Robotic myomectomy for large uterine myomas. Taiwan J Obstet Gynecol 2018;57:796–800.


22. Pluchino N, Litta P, Freschi L, Russo M, Simi G, Santoro AN, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot 2014;10:208–12.


23. Lönnerfors C, Persson J. Pregnancy following robot-assisted laparoscopic myomectomy in women with deep intramural myomas. Acta Obstet Gynecol Scand 2011;90:972–7.


24. Pitter MC, Srouji SS, Gargiulo AR, Kardos L, Seshadri-Kreaden U, Hubert HB, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int 2015;2015:967568.



25. Huberlant S, Lenot J, Neron M, Ranisavljevic N, Letouzey V, De Tayrac R, et al. Fertility and obstetrical outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot 2020;16:e2059.


26. Lundon D, Kelly B, Bouchier-Hayes D. 1518 surgeon morbidity does robot-assisted really cause less trauma to the operator? An international multiple surgeons’ opinion from experience of over 3,000 cases. J Urol 2012;187:e614–5.

27. Moawad GN, Tyan P, Paek J, Tappy EE, Park D, Choussein S, et al. Comparison between single-site and multiport robot-assisted myomectomy. J Robot Surg 2019;13:757–64.


28. Choi EJ, Rho AM, Lee SR, Jeong K, Moon HS. Robotic single-site myomectomy: clinical analysis of 61 consecutive cases. J Minim Invasive Gynecol 2017;24:632–9.


29. Kim M, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ. Robotic single-site myomectomy: a single-center experience of 101 consecutive cases. Int J Med Robot 2019;15:e1959.


30. Won S, Lee N, Kim M, Kim MK, Kim ML, Jung YW, et al. Robotic single-site myomectomy: a hybrid technique reducing operative time and blood loss. Int J Med Robot 2020;16:e2061.


31. Kim JJ, Choi C, Nam SH, Kim WY. Feasibility of reduced-port robotic surgery for myomectomy with the da Vinci surgical system. J Minim Invasive Gynecol 2017;24:926–31.


32. Lee NH, Lee SH, Kim WY. Comparison of reduced-port robotic surgery (RPRS) with conventional 2 port laparoscopy for myomectomy. Eur J Obstet Gynecol Reprod Biol 2020;247:181–5.


-
METRICS

-
- 2 Crossref
- 4,804 View
- 57 Download
- Related articles in Gyne Robot Surg
-
Asian Summit on Robotic Surgery 2022 gynecology tract meeting report2023 March;4(1)
Robotic surgery; into the era of common place2021 March;2(1)
Trends in robotic surgery in Korean gynecology2020 September;1(2)