 |
 |
- Search
| Gyne Robot Surg > Volume 4(2); 2023 > Article |
|
Abstract
Myomectomy is a surgical option for patients who wish to preserve fertility when removal of uterine fibroids is necessary. Robot-assisted laparoscopic surgery provides increased surgical dexterity during minimally invasive myomectomy (MIM) and permits greater accuracy with a layered closure of the uterine defect. However, uterine rupture following myomectomy has been reported more frequently with MIM than with laparotomy, including robot-assisted laparoscopic myomectomy (RALM). This article reviews the mechanisms of frequent uterine rupture following MIM and the optimal energy use during RALM.
Myomectomy is an important surgical option, other than hysterectomy, for those who wish to preserve fertility when the removal of uterine fibroids is necessary [1]. Due to the complexity of the suturing technique, laparotomy is typically required in difficult cases. Recently, minimally invasive surgery has become the standard approach for many gynecologic surgeries as it possesses several benefits, including reduced postoperative pain and bleeding [2]. The American College of Obstetricians and Gynecologists and American Association of Gynecologic Laparoscopists endorse a minimally invasive approach for myomectomy whenever feasible [3, 4]. Therefore, the number of minimally invasive myomectomies (MIMs) is increasing continuously [5]. However, uterine rupture following myomectomy has been reported more frequently in MIMs than in laparotomy [6]. There is no consensus regarding factors that increase the risk of uterine rupture after a minimally invasive surgery.
This article reviews the mechanisms of frequent uterine rupture following MIM and the optimal energy use during robot-assisted laparoscopic myomectomy (RALM).
Fibrotic changes in myometrial scars after myomectomy can result in myometrial rupture during pregnancy. Uterine rupture following an abdominal myomectomy is rare [7]. 15 cases of uterine rupture have been reported over 18 years after laparoscopic myomectomy [8]. However, there is no consensus regarding the factors that increase the risk of uterine rupture following MIM. Several possible mechanisms include (a) difficult surgical techniques in laparoscopic suturing of the myometrium and limitation of articulation of laparoscopic instruments, (b) failure to adequately suture myometrial defects, (c) lack of hemostasis with subsequent formation of a hematoma, and (d) excessive use of monopolar or bipolar electrosurgery with devascularization.
Robot-assisted devices continue to advance minimally invasive surgeries across a wide spectrum of surgical procedures. Advanced instrumentation, vision, and features, such as integrated table motion, enable surgeons to perform more complex procedures using a minimally invasive approach. The first three mechanisms that could possibly increase the risk of uterine rupture following MIM were resolved using a robotic system. However, electrosurgery is still required to achieve better hemostasis for hysterotomy and bleeding control.
A comprehensive review identified 19 cases of uterine rupture after laparoscopic myomectomy [8]. Among these, a monopolar electrosurgical device was used for hysterotomy. Furthermore, a bipolar device was used in more than half of the cases to achieve hemostasis alone or along with sutures. In another study that reported six cases of uterine rupture following laparoscopic myomectomy, monopolar and bipolar devices were used in all cases during hysterotomy and hemostasis [9].
Uterine rupture after RALM has also been reported previously. In this case, a cutting current in a low-energy setting was used for the initial hysterotomy [10]. The uterine fibroid was not degenerative, and the myometrial defect was closed using three layers of long-lasting absorbable sutures. The authors commented that the use of bipolar cautery as a method of hemostasis in the shelling out of the myoma could be a possible reason for the rupture. In another study, one case of uterine rupture was reported across 92 pregnancies following RALM [11]. A monopolar device was used only for hysterotomy, and the defect was closed using a multilayer absorbable suture. In this study, monopolar devices were used in 34% of the cases, including those of uterine rupture. In contrast, a harmonic scalpel was used for hysterotomy in other cases. The authors stated that they used only a harmonic scalpel for uterine incision after this study. A randomized controlled study comparing the use of a harmonic scalpel and electrosurgery in laparoscopic myomectomy showed that the harmonic scalpel was superior in terms of reduced bleeding and shorter operating time [12]. Many surgeons prefer using harmonic scalpels during laparoscopic myomectomies. Owing to the risks of electrosurgery, ultrasonic energy can also be used with robots to perform hysterotomies. However, robotic harmonic shears are unable to articulate in a manner similar to all other robotic instruments, thus losing two of the seven degrees of freedom of movement. They only work in a straight-forward direction and rotation, without endo-wrist function, making their proper placement more difficult during surgery. Additionally, the use of a harmonic scalpel does not guarantee protection from thermal damage. Uterine rupture during pregnancy following laparoscopic myomectomy using a harmonic scalpel has been reported even though the surgeon did not use any other electrosurgical device [13]. Accordingly, the adequate use of electrosurgical devices to reduce myometrial vascular damage is more important than the type of surgical device used. Therefore, we must determine the details of the electrosurgical device used in the robotic surgical system.
The 4th generation daVinci surgical systems adapted ERBE VIO® dV 2.0 (ERBE USA, Marietta, GA, USA; Intuitive, Sunnyvale, CA, USA) as an electrosurgical generator (Fig. 1). This is consistent with monopolar, bipolar, and advanced bipolar energy options [14]. Several monopolar energy sources are available (Table 1). In the monopolar option, there are two overarching waveforms; one designed to cut or transect tissues (“cut” modes), and the other designed to achieve more hemostasis while still being able to transect tissue (“coag” mode). It includes additional waveforms such as “swift coag” and “forced coag” that provide blended waveforms which respond differently to tissue resistance as they are being used. This system also requires the selection of a “tissue effect” as well as a “power limit.” Myriad possible tissue effects are illustrated in Fig. 2.
The manufacturer also provided an energy-output reference chart (Fig. 2). The manufacturer commented that the table only approximates the constant power output of the electrosurgical unit. Additional changes may be necessary to achieve the desired tissue effect; therefore, surgeons should attempt to determine the adequate mode and power for the procedures they perform. There is no consensus regarding the appropriate mode or power for each procedure. For instance, during RALM, the hysterotomy can be performed with classic coag mode with “effect 1, power limit 80’” to reduce lateral tissue thermal damage. However, during robot-assisted total laparoscopic hysterectomy, swift coag mode with “effect 3, power limit 100” can be appropriate for tissue coagulation to achieve sufficient hemostasis. An interesting study evaluated whether bipolar electrosurgery could be performed over the suture site without compromising the tensile length of the suture material during laparoscopic myomectomy [15]. The authors concluded that bipolar electrosurgery within two seconds with power under 40 watts is safe, and the utilization of a bipolar device resulted in reduced blood loss, shorter surgical time, and shorter hospital stay compared to non-users.
Several types of medical interventions can contribute to minimizing the use of electrosurgical devices during RALM. First, the use of gonadotropin-releasing hormone agonists (GnRHas) before myomectomy has been extensively studied. According to a Cochrane review of 14 randomized controlled trials (RCTs) of GnRHas, the preoperative use of GnRHas had a significant positive impact on preoperative hemoglobin and hematocrit levels and reduced intraoperative estimated blood loss (EBL) [16]. Given the small number of MIMs included in this analysis, there is little evidence to support the benefit of GnRHas in reducing blood loss in MIMs compared to its significant effect on open myomectomy.
To elucidate the effect of GnRHa treatment before MIM, three RCTs were evaluated in a meta-analysis [17]. In this analysis, pretreatment with GnRHas resulted in a significant decrease in the intraoperative EBL and high postoperative hemoglobin concentrations. However, GnRHa pretreatment did not reduce the operating time, perhaps because of its degenerative impact on the myoma, which led to increased surgical difficulty. Accordingly, surgeons should consider whether the benefit of GnRHas on hemostasis outweighs its impact on operating time, technical challenges of myomectomy, and common side effects, such as vasomotor symptoms, vaginal dryness, and bone loss.
Vasopressin has a role in regulating homeostasis and peripheral vascular resistance. A prospective study of nearly 300 women undergoing laparoscopic myomectomy demonstrated that vasopressin injection reduced EBL and higher postoperative hemoglobin levels compared to those with pedicle ligation [18]. In that study, vasopressin injection with pedicle ligation was more effective in achieving hemostasis. However, the use of vasopressin can result in severe cardiopulmonary complications even in healthy individuals, including hypotension, bradycardia, cardiac arrest, and pulmonary edema [19]. Therefore, vasopressin concentration should be as low as 0.05-0.3 units/mL. Additionally, the anesthesia staff should be notified when vasopressin is injected to be aware of the occurrence of possible side effects.
Oxytocin infusion is usually used to achieve hemostasis after delivery and to prevent postpartum hemorrhage because of its physiological role in stimulating uterine smooth muscle contraction. Therefore, its use in myomectomies has been evaluated in several studies. In an RCT involving 100 women undergoing open or vaginal myomectomy, oxytocin treatment did not show promising results [20]. In contrast, in a prospective study, a significant reduction in EBL and transfusion rates was observed during laparoscopic myomectomy with oxytocin treatment [21]. The efficacy of oxytocin infusion can be altered by dosing differences or timing of administration [22].
Use of different types of hemostatic agents to aid in hemostasis during myomectomy has also been investigated. In a prospective randomized study, the application of a gelatin-thrombin matrix (FloSeal; Baxter Healthcare Corp., Fremont, CA, USA) resulted in significantly decreased EBL during surgery compared to that in the placebo group [23]. It was also associated with decreased intraoperative transfusion, higher postoperative hemoglobin levels, reduced postoperative blood loss, and shorter hospitalization duration. However, further studies are warranted, owing to the small sample size of the cohort in this study.
Another agent, a fibrin sealant-coated suture (Tisseel; Baxter Healthcare Corp., Deerfield, IL, USA) used during laparoscopic myomectomy, also showed efficacy in terms of reduced mean time to achieve hemostasis, operating time, EBL, and postoperative hemoglobin levels [24]. Adequate utilization of commercial hemostatic agents can help reduce unnecessary electrocoagulation, thereby preventing poor myometrial healing.
Robot-assisted laparoscopic surgery provides increased surgical dexterity during MIM and permits greater accuracy with a layered closure of the uterine defect. However, uterine rupture after RALM has also been reported. Extensive use of electrosurgical devices may be a major reason for this discrepancy. Therefore, surgeons need to understand the details of the electrosurgical device of a robotic surgical system and attempt to determine their own appropriate modes and powers. Excessive use of electrocautery for hemostasis should be avoided because it results in poor vascularization and necrosis of the myometrium, impaired scar healing, and decreased tensile strength of the myometrium. Expeditious suturing of the myometrium is preferred to achieve hemostasis rather than indiscriminate electrocauterization. Various preoperative and intraoperative options are available to decrease blood loss, transfusion requirements, changes in postoperative blood count, and duration of hospitalization. In conclusion, surgeons should put in their best efforts at every step of RALM.
Fig. 1.
ERBE VIO® dV 2.0 system was adapted as an electrosurgical generator for the 4th generation daVinci systems.

Fig. 2.
Monopolar modes on ERBE VIO® dV and energy output reference chart. Depiction of tissue effect with different monopolar modes on the integrated ERBE VIO® dV generator.
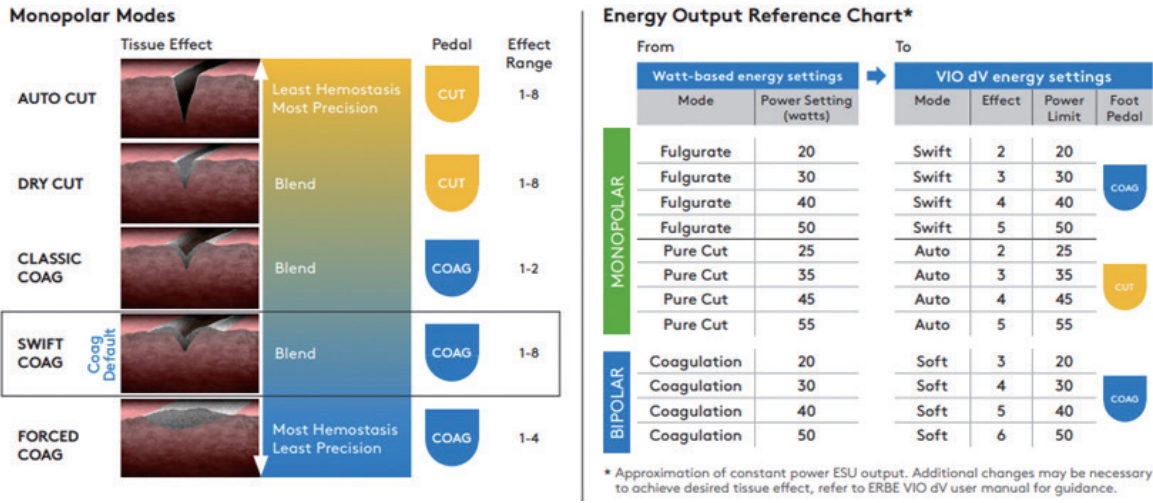
Table 1.
Monopolar energy modes
References
1. Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol 2011;117(2 Pt 1):256–66.


2. Kim S, Min KJ, Lee S, Hong JH, Song JY, Lee JK, et al. Robotic single-site surgery versus laparo-endoscopic single-site surgery in ovarian cystectomy: a retrospective analysis in single institution. Gyne Robot Surg 2020;1:21–6.

3. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: robotic-assisted laparoscopic surgery in benign gynecology. J Minim Invasive Gynecol 2013;20:2–9.


4. American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice; The Society of Gynecologic Surgeons. Robot-assisted surgery for noncancerous gynecologic conditions: ACOG COMMITTEE OPINION, Number 810. Obstet Gynecol 2020;136:e22–30.

5. Zaritsky E, Le A, Tucker LY, Ojo A, Weintraub MR, Raine-Bennett T. Minimally invasive myomectomy: practice trends and differences between Black and non-Black women within a large integrated healthcare system. Am J Obstet Gynecol 2022;226:826.e1–11.


6. Kim MS, Uhm YK, Kim JY, Jee BC, Kim YB. Obstetric outcomes after uterine myomectomy: laparoscopic versus laparotomic approach. Obstet Gynecol Sci 2013;56:375–81.



7. Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol 1966;94:571–6.


8. Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol 2010;17:551–4.


9. Lu B, Wang Q, Yan L, Yu K, Cai Y. Analysis of pregnancy outcomes after laparoscopic myomectomy: a retrospective cohort study. Comput Math Methods Med 2022;2022:9685585.



10. Markuly SN, Miller CE, Szela K. Uterine rupture after robotic-assisted laparoscopic myomectomy. CRSLS 2014;e2014.00208. https://doi.org/10.4293/CRSLS.2014.00208.

11. Pitter MC, Gargiulo AR, Bonaventura LM, Lehman JS, Srouji SS. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod 2013;28:99–108.


12. Litta P, Fantinato S, Calonaci F, Cosmi E, Filippeschi M, Zerbetto I, et al. A randomized controlled study comparing harmonic versus electrosurgery in laparoscopic myomectomy. Fertil Steril 2010;94:1882–6.


13. Yazawa H, Takiguchi K, Ito F, Fujimori K. Uterine rupture at 33rd week of gestation after laparoscopic myomectomy with signs of fetal distress. A case report and review of literature. Taiwan J Obstet Gynecol 57:304–10.


15. Sol ES, Hong SY, Oh HK, Kim AS, Sin JI, Choi YS. Can bipolar electrosurgery be performed over suture sites without compromising tensile strength of suture material during laparoscopic myomectomy? J Minim Invasive Gynecol 2011;18:157–63.


16. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev 2001;(2):CD000547.

17. Chen I, Motan T, Kiddoo D. Gonadotropin-releasing hormone agonist in laparoscopic myomectomy: systematic review and meta-analysis of randomized controlled trials. J Minim Invasive Gynecol 2011;18:303–9.


18. Lin XN, Zhang SY, Fang SH, Wang MZ, Lou HY. [Assessment of different homeostatic methods used in laparoscopic intramural myomectomy]. Zhonghua Yi Xue Za Zhi 2008;88:905. –8. Chinese.

19. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol 2009;113(2 Pt 2):484–6.


20. Agostini A, Ronda I, Franchi F, Bretelle F, Roger V, Cravello L, et al. Oxytocin during myomectomy: a randomized study. Eur J Obstet Gynecol Reprod Biol 2005;118:235–8.


21. Wang CJ, Lee CL, Yuen LT, Kay N, Han CM, Soong YK. Oxytocin infusion in laparoscopic myomectomy may decrease operative blood loss. J Minim Invasive Gynecol 2007;14:184–8.


22. Hickman LC, Kotlyar A, Shue S, Falcone T. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol 2016;23:497–504.


-
METRICS

-
- 0 Crossref
- 1,617 View
- 11 Download
- Related articles in Gyne Robot Surg
-
Robot-assisted laparoscopic adenomyomectomy: A review of literature2021 March;2(1)
Robotic-assisted laparoendoscopic single-site total omentectomy2020 March;1(1)





