Overcoming the limitations of single-site robotic myomectomy via the gradual turning out method without an accessory port, and a know-how
Article information
Abstract
Robotic surgery has advantages in myomectomies, which are a technically complex surgery. Multi-site robotic myomectomy (MRM) is widely used and is a safe, minimally invasive surgery. Single-site robotic myomectomy (SSRM) is further expected to provide potential advantages such as better cosmesis and easier fibroid removal. Compared with MRM, SSRM has technical limitations, including a restricted range of motion, especially for large-sized myomas; less powerful suturing in thick myometrium; and non-articulating instruments. To overcome these limitations, additional ports are used, larger skin incisions are made, and the procedure is combined with laparoscopy. However, these adjustments may neutralize the advantages of a single-port surgery. Here we describe our novel gradual turning out method, which enables us to do SSRM without adjustments. Additionally, we introduce know-how on how to accomplish an SSRM.
INTRODUCTION
Uterine myomas are the most common gynecologic disease [1]. Myomectomies are frequently performed in Korea, with a rate of 14.6±0.1 per 10,000 patients, and the conversion rate from a myomectomy to a hysterectomy is 2.9±1.1 per 10,000 patients [2]. The mortality rate after a myomectomy is 0.013%, which is substantially lower than other surgeries [2]. Cosmetics are an important concern to patients, and the impacts the decision females make in undergoing minimally invasive surgeries.
Because of its advantages, laparoscopy has replaced abdominal myomectomy since it is a minimally invasive surgery [3]. However, laparoscopic myomectomy (LM) is a technically demanding operation because of the technical complexity and steep learning curve [4]. With the evolution of technology, robotics has been used during myomectomies. The use of robotics during surgery has many advantages, including three-dimensional stereoscopic vision and magnification, articulating instruments, tremor-filtration, and reduced surgical fatigue. Recently, multi-port robot-assisted myomectomy (MRM) has been shown to be safe and feasible [5-8]. Accordingly, single-site robotic myomectomy (SSRM) is expected to provide further potential advantages, including better cosmesis and higher patient satisfaction [9]. The feasibility and safety of an SSRM have been reported [9-14]; however, most of these studies used accessory ports or longer skin incisions, and these adjustments neutralized the advantages of single-port surgeries.
Here we describe the novel gradual turning out method (GTOM) for performing an SSRM, which is safe and feasible [15]. An accessory port or a longer incision is not required in GTOM, which yields better cosmetic results. In addition, we summarize our personnel know-how to successfully accomplish an SSRM.
ADVANTAGES OF SSRM
Single site LM through the umbilicus provides the bene- fits of minimally invasive surgeries, including easier fibroid removal due to a large umbilical incision, better cosmetics, and less pain, than the traditional multi-port LM [16-18]. However, surgeons experience less freedom of movement and conflict of instruments due to their proximity to each other. The single-site robotic system does not have the conflict of instruments due to the curved shaft of the trocars and instruments. Additionally, the single-site robotic system still has the benefit of a robotic system, including better visualization with a three-dimensional stereoscopic vision and magnification and reduced surgical fatigue by using an articulating instrument with tremor-filtration.
Although SSRM is still in the initial learning curve, recent reports showed its feasibility [9-15]. SSRM was shown to have comparative surgical outcomes in terms of estimated blood loss, hospital stay, and perioperative complications to traditional MRM [9, 13]. Additionally, compared with LM, SSRM yields similar perioperative outcomes, including length of hospital stay, estimated blood loss, decrease in the hemoglobin level, transfusion rate, and postoperative pain [15]. However, operative time was significantly longer in SSRM for myomas located at the anterior wall, singleton myomas, and myomas less than 10 cm, compared with LM. In contrast, the surgical time was not different between SSRM and LM for myomas located at the posterior or lateral wall, multiple myomas, and myomas equal to or greater than 10 cm. These findings suggest that SSRM has advantages for difficult myomectomies. These advantages have facilitated the use of SSRM.
INSTRUMENT LIMITATIONS OF SSRM AND PERSONNEL KNOW-HOW
However, SSRM has some drawbacks compared with MRM. These include a restricted working space due to the long, fixed length of the curved trocar, especially in cases of large-sized myomas; less powered suturing of thick myometrium due to the semi-rigid shaft of the needle driver; and the inability to use advanced energy instruments, or non-articulating instruments other than needle drivers [12,19]. Surgeons could partially overcome the two former limitations with some know-how; however, the latter two limitations are technological issues.
Limited working space is especially true for large-sized myomas or female with short statures where the uterus is located close to the port site. The limited space results in a short distance between the tumor and instruments, compromising the instrument’s functional space. In these cases, since the instrument cannot be retracted further back than the tip of the trocar, the trocars should be pulled all the way back to make more space. When the trocars are fully retracted, the distance between both trocars is marginal, and the two are placed in a line, resulting in a collapse of the intraperitoneal triangulation. This collapse results in surgical discomfort and produces the same limitations as a single site laparoscopic surgery. However, the trocars must be gradually pushed in during the operation since the uterus is diminished by the peeling of the pseudomembrane off and the contracting myometrium.
The other limitation of less powered suturing for thick myometrium is a result of the instrument shaft being semirigid, which enables it to pass through the curved trocar. During suturing of bulky tissue, the shaft is easily bent, resulting in the reduction of suturing power. In this case, two methods are recommended. One recommendation is that the trocar of needle driver be fully pushed in, and suturing should be performed using the power of the rigid trocar. This technique provides more power for suturing and is especially helpful in total hysterectomies, where the vaginal cuff is far from the trocar. The trocar is rigid and should be pushed in more than the standard level to provide more powerful suturing. The second recommendation is that the thick myometrium could be sutured in two or three layers, which reduces the amount of myometrium that needs to be sutured.
ALTERNATIVE METHODS AGAINST LIMITATION OF SSRM, SHOWN IN THE LITERATURES
To overcome these limitations, surgeons use an accessory port, make larger umbilical incisions, and incorporate laparoscopic technology in the surgery [9,10,12,13,20,21]. In the first alternative method, an assistant’s port is used for claw forceps, single tooth tenaculum, or a myoma screw to assist in holding and keeping traction on the myoma [9,12,13]. Although steady traction is necessary for effective separation of the leiomyoma from the surrounding tissues, the traction in SSRM is relatively weak because it is achieved by stabbing with a monopolar hook or by pushing with bipolar forceps in the opposite direction of the operation point and is not strong enough to maintain the traction. An additional port operated by an assistant provides better traction; however, these surgeries are not topically single-site surgeries.
Another alternative method is to use a larger umbilical incision, such as a 3-4 cm incision [10]. This study showed that a larger incision allows for three trocars, one camera and two rigid instruments used for the multi-port robotic surgery, instead of the single port instruments, which have the limitations in the range of motion. The use of multi-port robotic instruments yields maximal benefits. The larger incision can be used in patients whose umbilical habitus is big enough to be cosmetically contained within the umbilicus.
A third alternative method is to incorporate laparoscopic technologies [20,21], which have the advantages of integrating the two systems, including extraction of the myoma from the uterus performed by the single-site laparoscopy and uterine defect repair by the single-site robotic system. This method reduces operation time, blood loss, and conversion rate to multi-port laparoscopy compared with SSRM.
However, these adjustments may neutralize the advantages of single-port surgeries. The GTOM method does not need an accessory port, longer incision, or combination with laparoscopy or multi-port robotic system and thereby yields better cosmetic outcomes.
SURGICAL PROCEDURE FOR GTOM IN SSRM
Abdominal entry
A 2.0-2.5 cm vertical midline incision through the center of the umbilicus was made with an open Hasson approach [15,18]. The peritoneum was opened with Kelly forceps through the natural defect at the umbilical center. Fasciotomy was done through the gap between the opened Kelly forceps, and the peritoneum was incised further. The port was inserted through the umbilical wound (Fig. 1).
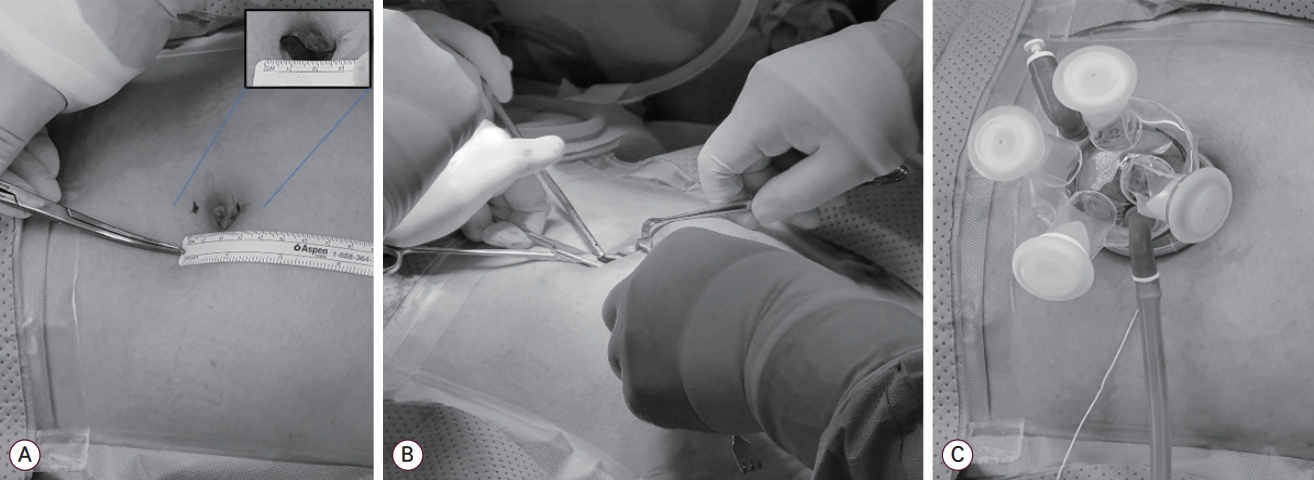
Abdominal entry and port equipment. (A) A 2.0-2.5 cm sized midline vertical skin incision was made. (B) The umbilical opening was made with the open Hasson technique. The fasciotomy was done through the gap between the opened Kelly forceps. (C) A Gloveport (IROOINS, Seoul, Korea), which we prefer, was equipped.
Before docking
After the patient was placed in the Trendelenburg position, the abdominal and pelvic cavities were explored. Three laparoscopic gauzes were placed in the right paracolic gutter, near the right round ligament and posterior of the culde-sac. During the procedure, the gauzes were useful to control minor bleeding by compressing, and to visualize the surgical area by wiping blood clots. The suture materials were placed in the right paracolic gutter. Vasopressin at a concentration of 0.25 U/mL was infiltrated into the subcapsular area. Electric marking of the incision line was helpful for larger-sized myomas, in which the entire mass was not visible under the robotic magnification (Fig. 2A).
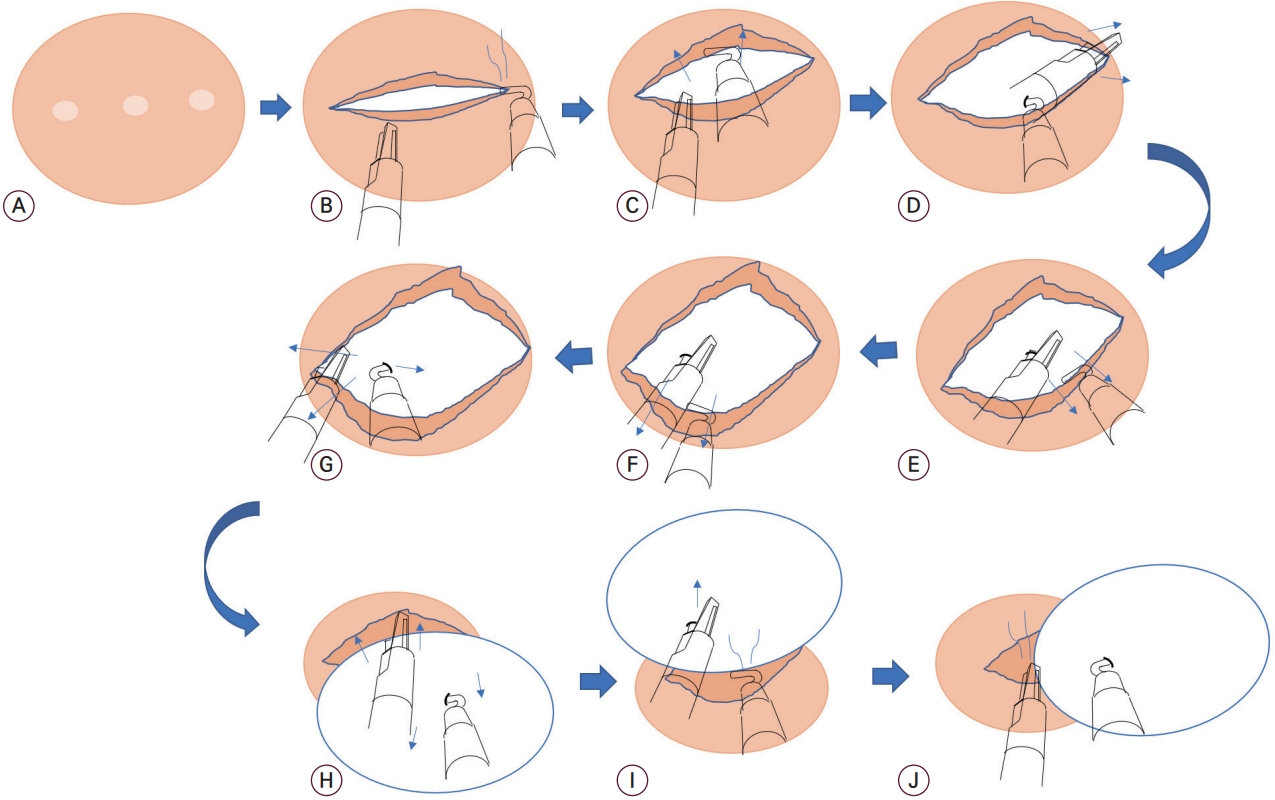
The gradual turning out method (GTOM). (A) An incisional line was marked with bipolar cautery. (B) An incision at the serosa and surrounding myometrial shell was made with a monopolar hook. (C) The uterus was held by grasping the incisional myometrium with bipolar forceps, and the myometrium was peeled at the other side by pushing with the monopolar hook. (D) The myoma was held by stabbing it with the monopolar hook and peeling the pseudomembrane with bipolar forceps. (E, F) The myoma was held by stabbing it with bipolar forceps and peeling the pseudomembrane with the monopolar hook. (G) The myoma was hooked on the right side and the left side of the myoma was separated. (H-J) Once the diameter of the myoma was out, the myometrium was contracted, and the uterus was shrunk. At this moment, because the myoma obstructed the operation field, the myoma was pushed aside and was continuously separated from the uterus by coagulating the attached tissues.
After docking
An 8.5 mm, 30° stereo-endoscope was introduced through the robotic trocar, and two curved cannulas were introduced in cross direction under direct visualization. Fenestrated bipolar forceps and a monopolar hook were inserted. An intravenous line extension was inserted for evacuation of surgical smoke [15], which worked better than we thought it would. This line could be used for suction and irrigation by switching the three-way valve.
Enucleation of myoma
To reserve the working space for the instruments, the uterus was fully pulled down, and the long-curved trocars were properly pulled out. A horizontal incision at the serosa and the surrounding myometrial layer was made using a monopolar hook (Fig. 2B). During the incision of the large-sized myoma, a semi-circular incision was used. The mass was pushed with bipolar forceps to establish more space for the monopolar hook’s motion. The myoma was held by stabbing it with the hook, and the surrounding pseudo-membrane was peeled with bipolar forceps (Fig. 2C-G). This procedure was gently repeated in all directions of the incision, and the mass was gradually turned out. The bleeding was strictly controlled to avoid contaminating the field with blood. When the longest part of the mass was turned out, sudden bleeding occurred from the decompressed blood vessels, which were compressed by the mass but was well controlled with gauze compression and electrocauterization. The mass was pushed aside, and the surrounding tissue was continuously coagulated and separated (Fig. 2H and I). A major part of the mass was removed from the uterus, and the stump was separated (Fig. 2J). Coagulation was used throughout the surgery since the bleeding from torn vessels can be hard to control. The specimen was placed in the paracolic gutter.
Suturing of myometrium
Suturing was not different from other SSRM [15]. The monopolar hook was exchanged for a wristed needle driver. The rigid trocar was pushed in as much as possible to get more suturing force. Suturing time was a major point of blood loss because the incised tissues’ blood vessels were exposed. The crater of the mass was quickly sutured to minimalize the blood loss, and then the uterine myometrium was reapproximated layer by layer for two layers. Continuous running barbed sutures were used. The serosa was repaired in a running “baseball” fashion.
Specimen retrieving
The robot system was undocked and the stagnant blood was sucked out with the laparoscopic suction instrument. The specimen was retrieved into the Endopouch bag and was brought up to the umbilical opening via morcellation with a scalpel.
THE ADVANTAGE OF GTOM
SSRM with GTOM offers several advantages. First, an additional port for a screw or forceps are not needed; however, myoma enucleation takes more time in GTOM than other methods, which involve direct myoma traction with a screw or forceps. The effective bleeding control in this method made for a clean operation field, which is critical in minimally invasive surgeries. Second, the parallel arrangement of the two instruments made by pulling them back did not interrupt the GTOM since the instruments work forward and back or up and down, not sideways. The damage to the skin edge was a concern due to the excessive traction; however, the use of a 20-25 mm incision did not result in such injuries. Third, an intravenous extension line was used to evacuate surgical smoke and to vacuum blood.
CONCLUSIONS
SSRM is a unique surgical approach and benefits the patients. GTOM is a feasible approach to facilitate SSRM without any alterations, which neutralizes the advantages of single-port surgeries.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A2C1003536).